Review Process
Oversight by the IACUC starts with the IACUC protocol. Through the protocol review process, the IACUC draws upon the collective experience of its members to advise PIs on project feasibility in addition to regulatory compliance issues related to the PI’s intended animal use. The IACUC meets twice each month to review protocol submissions.
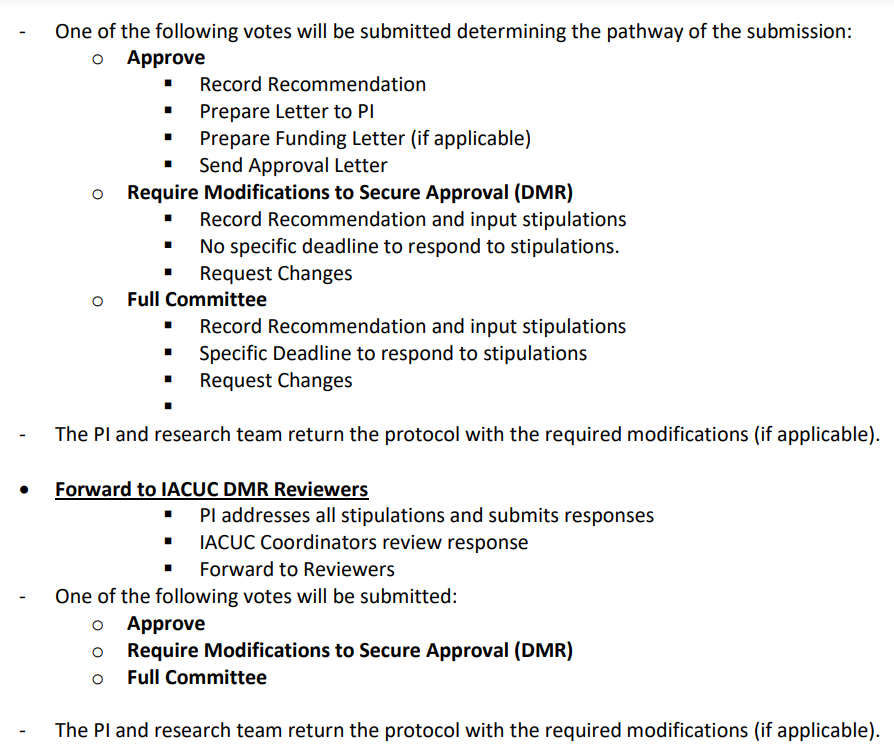
IACUC New Protocol Review Process
Protocol Synopsis Forms, when submitted to the IACUC by the submission deadline will be reviewed during that month.
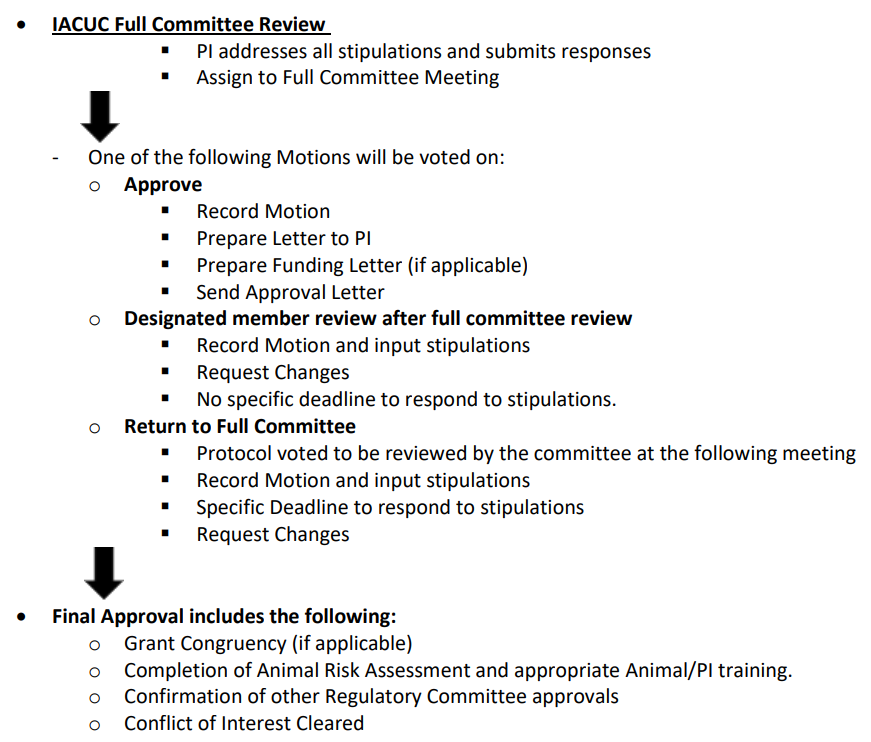
Following a pre–review meeting mid–month, the committee will send a list of questions/stipulations to be addressed by the PI. The PI has approximately one week to address these questions/stipulations before the full committee meeting.
If all questions and stipulations are answered to the committee’s satisfaction, committee approval will be granted, notification of external agencies will take place, and animal work can begin.
IACUC New Protocol Review Process
Release of Funds and Procurement of Animals
Administrators and staff have been instructed not to release funds or process animal orders unless an approval protocol number or verification is present and all stipulations attached to the protocol have been addressed.
Annual Reviews
Even though you may be approved for a multiple year project, the IACUC must review each protocol on an annual basis as required by federal regulations. You will be sent a reminder two months before the anniversary of the original approval date. Updated information and any changes in the animal use protocol must be supplied on the Continuing Review form. Approvals will be extended for another year if there are no major changes in the protocol.
Submitting a Modification (Amendment) to an Approved Protocol
If there are any changes to the protocol during the course of the research project or upon annual renewal, an amendment should be submitted to the IACUC for approval using the Continuing Review form.
The following minor changes to an approved animal use and care protocol can be submitted to the IACUC in the form of an amendment:
- a) change of title
- b) change of funding agency
- c) addition or deletion of personnel
- d) change of the animal strain
- e) change or addition of minor procedures
- f) increase in animal numbers with justification
- g) addition of a new co?investigator
- h) change of research facility
- i) change in the method of euthanasia
- j) change in anesthetic or drug dosage
The following major changes generally require a submission of the entire protocol (a completed Protocol Synopsis Form) for IACUC approval.
- a) change of species
- b) change of drug classification which will be administered to animals
- c) change from type A to type B or C procedures (refer to the protocol instructions (link to protocol instructions) for information on procedure categories)
- d) addition of major procedure (i.e. survival surgical manipulation or other procedures requiring anesthesia with recovery, procedures involving hazardous or infectious agents provided the PI also shows evidence of approval from the appropriate safety committee)
- e) transfer of the protocol to a new principal investigator
The IACUC reserves the right to request a full protocol if deemed necessary for any protocol.
3-year Renewals
The Public Health Service policy requires a complete review of activities every three years. To do this, a completed Protocol Synopsis form must be submitted and approved by the IACUC.